Smart watches, fitness trackers, smart clothing, smart medical attachments, data gloves – the market for wearable sensor electronics in health care, fitness and wellness is growing rapidly.
The field of wearable electronics consists of several components – sensors, actuators, electronics and power storage or power generation – which all have to be combined into one, miniaturized device design. Whereas the first generation consisted mostly of detachable components, the second generation is moving towards textile-embedded sensors, actuators, and therapeutic solutions (if you want to see how broad this field already is, take a look at our collection of Nanowerk Spotlights on wearable electronics). Also, a lot of the more recent devices rely on nanomaterials and/or nanotechnology enabled design.
One particular area of wearable sensor technology that has seen a lot of research interest recently concerns face masks. For instance, we already reported on a high-comfort smart face mask that checks for proper fit and monitors coughs or a FFP2 face mask that sends a mobile alert when CO2 limits are exceeded.
However, wearable biosensing devices are energy-hungry because of the growing demand for multiparameter detection, complex data processing, and real-time wireless data transmission.
In this regard, (bio)fuel cells have proven successful in the past for self-powered glucose sensors on a laboratory platform. The major challenge is to realize energy-autonomous wearable biosensing systems that contain practical and efficient energy harvesters to continuously provide power as well as show signals for biosensing purposes such as for instance glucose detection.
A research team in Thailand set out to solve the energy supply problem while adding an extra biosensing function to a single face mask. Moreover, they worked on reducing the complexity and cost of fabrication.
Their recent paper in Sensing and Bio-Sensing Research (“Wearable energy devices on mask-based printed electrodes for self-powered glucose biosensors”) describes the first example of self-powered bioelectronics on a mask that can measure the biological glucose signal with continuous energy.
In their work, the team demonstrated a 3-in-1 mask device that can 1) harvest energy (a biofuel cell); 2) store energy (a supercapacitor); and 3) indicate glucose concentration (a biosensor).
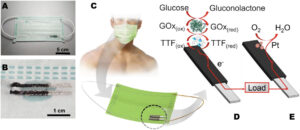
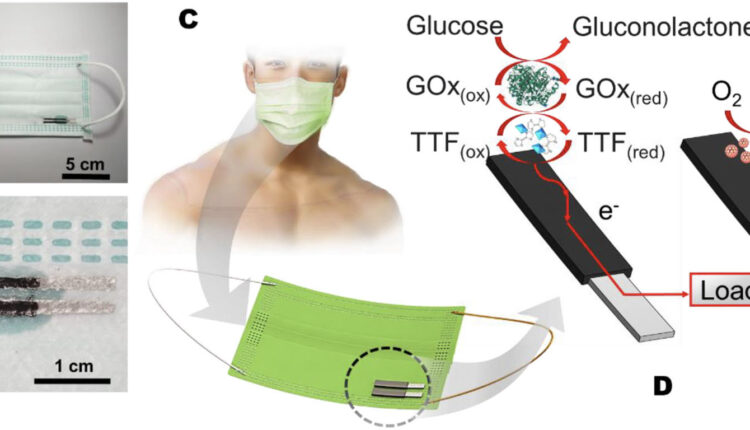
Schematic illustration of the mask-based bioelectronic device for harvesting energy from glucose and self-powered monitoring glucose. (A) Photograph of the mask-based bioelectronic. (B) Zoom-in photograph of the electrodes. (C) The concept of the mask-based bioelectronic device using sweat containing glucose to produce electricity and a self-powered signal. (D–E) The components of the mask-based bioelectronic device and the redox reactions that occur on (D) the bioanode and (E) the cathode. (Reprinted from doi:10.1016/j.sbsr.2022.100525 under a Creative Commons CC-BY 4.0 license)
“We provide the first description of mask-based enzymatic biofuel cells using glucose and natural oxygen,” Itthipon Jeerapan, an assistant professor and a research principal investigator at Prince of Songkla University in Thailand, and the paper’s first author, tells Nanowerk. “The small biofuel cells can harvest energy from the sweat on a person’s face and indicate the level of the analyte that tracks health and nutrition.”
The engineering breakthrough that allowed the researchers to achieve the 3-in-1 mask is based on a screen-printed electrode, which is flexible on the mask and contains active functional materials to help the catalytic reaction (for energy harvesting and biosensing) and the electrical capacitance (for storing energy in a supercapacitor module). This allows for a self-sustainable device as well as for scaling up the fabrication process to meet commercial requirements.
Jeerapan points out that the enzyme – glucose oxidase – functionalized on the active and flexible electrode is key to the reaction to extract the electrons, i.e. generate electricity. The extracted electricity is proportional to the glucose concentration and therefore can be an analytical signal for self-powered detection.
In addition to energy harvesting and biosensing, the nanocomposite with conducting polymers on the electrode can help store the electricity for this mask-based biosupercapacitor.
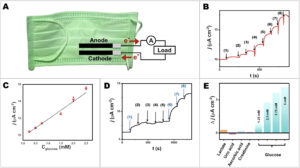
Self-powered glucose biosensors. (A) illustration of the self-powered biosensing electronic system. (B) The calibration plot against the concentration of glucose obtained from self-generated current responses using the mask-based glucose biosensor. The current was observed at a constant load between electrodes of 98.7 kΩ (no applied potential from an external source) upon the addition of glucose). ((1–8): 0.25, 0.50, 0.75, 1.50, 2.00, 2.50, 5.00, and 10.00 mM glucose). (C) The self-powered current output upon increasing the glucose concentrations. The error bars are standard deviations (n = 3). (D) The selectivity test showing the current output to (1) 1.25 mM glucose (2) 28 mM lactate, (3) 118 µM uric acid, (4) 20 µM ascorbic acid, (5) 168 µM creatinine, (6) 2.50 mM glucose, (7) 3.75 mM glucose, and (8) 5.00 mM glucose with no external applied potential. (E) The corresponding plot showing the self-powered responses toward glucose and other substances. (Reprinted from doi:10.1016/j.sbsr.2022.100525 under a Creative Commons CC-BY 4.0 license)
“We got our inspiration from the current situation that we all have to wear masks,” says Jeerapan. “So we set out to add extra functions to a conventional face mask to generate electricity while providing support for wearers to monitor their stamina and fitness or manage their nutrition consumption.”
The researchers are now looking to push the limits of what this platform can do. They will combine their system with other sensors and energy devices to fabricate devices for detecting multiple analytes in a self-powered and self-sustainable fashion. They are also working on a new way to expand this concept to other biomolecule targets (including toxic pollutants and pathogens) that could come in contact with our face.
“The main challenge of this field relies on creating digital and Internet-of-Things (IoT) systems or giving feedback to the user,” Jeerapan concludes. “An example is a glucose sensor that closes the loop with insulin pumps just like in our bodies, which are designed with a closed-loop communication system between blood sugar sensors and the pancreas (responding to these levels with the hormones insulin and glucagon).”